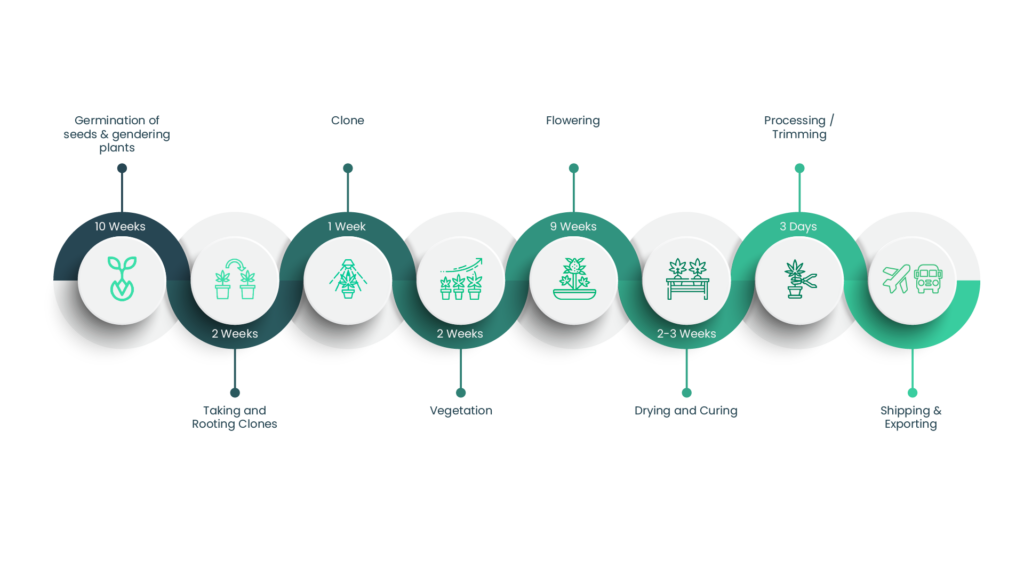
Production
At CINV, we meticulously craft premium medical cannabis cultivars, tailored for therapeutic benefits. Our advanced South African facilities ensure we have control and oversight on every step, from seed to sale.
Cultivation Expertise
With over six decades of combined cultivation experience, our team has a deep understanding of everything about the cannabis plant, from ideal structure to cannabinoid levels, terpene profiles, and flavour.
We harness cultivation science, consumer data and continuous innovation to address diverse patient needs. We have extensive knowledge of award-winning cultivation techniques, and the US legacy market, thus setting a new benchmark for cultivation practices.

Production Process

Testing
Once harvested, samples undergo robust testing in an EU-GMP certified third-party testing facility:
- Terpenes and cannabinoids
- Heavy metal accumulation
- Fungals, harmful bacteria, and pathogens
Strategic Partnerships
We partner with additional out-grow facilities to escalate our production capacity, onboarding them into our strategic framework. To discuss partnership opportunities, please get in touch.


End-to-End Quality Control
Our team directly oversees every harvest and ensures rigorous testing and compliance are undertaken through our third-party partners. Our seed-to-heal framework includes EU-GMP-certified processing, export, and distribution, via either a schedule 2 narcotics-certified dispensary or registered CQC clinics.